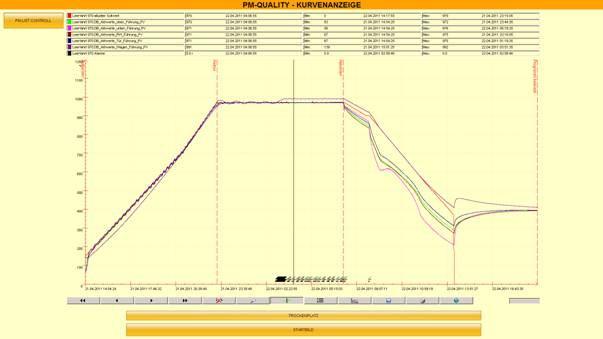
FDA-compliant data recording
A combination of SIEMENS software components WinCC/WinCCF and PM quality ensure data recording that complies with the requirements of the FDA (US Food and Drug Administration). Changes to the systems are recorded in an audit trail using an accompanying signature and time stamp. This protects the written data from being accessed from outside the system by integrating electronic symbols and symbol series. This ensures the necessary legally binding nature of the recorded data, which can be considered equivalent to a hand-written signature, thereby guaranteeing reliable traceability in the production process.